

九月启新,共探前沿。晶云药物将携创新技术,亮相九月多项海内外、线上线下行业会议活动,期待与行业各位同仁相约中国上海、美国波士顿以及费城,共话药物研发前沿议题。
行业会议
01
SIT 2025第七届小分子药物创新峰会
9/17-18 | 上海
请点击查阅详情 ↑
SIT 2025第七届小分子药物创新峰会将于2025年9月17-18日在上海盛大召开。此次大会将汇聚70多位业内顶尖的化学药物专家和1000+行业同仁,聚焦减重药物的突破性研究、多肽药物的最新进展以及不可成药靶点的攻克。
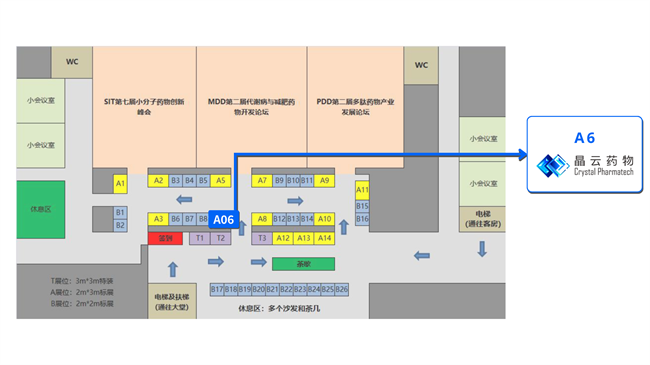
晶云药物将在A6展位期待各位莅临,同时晶云星空(晶云药物CDMO事业部)CEO马德成博士将围绕《PROTAC口服制剂的研发策略》为大家带来技术分享。
时间:2025年9月17-18日
展位:A6
2025年9月17日
14:30-15:00
《PROTAC口服制剂的研发策略》
介绍开发PROTAC口服制剂的挑战,包括如何增加溶解度,渗透性和提高生物利用度等,并用可行性研究和IND开发实际案例进行详尽的分析

马德成博士
晶云星空(CDMO事业部)CEO
拥有25年跨国药企的制剂研发及外包管理经验,曾在默克美国和默沙东中国工作22年。专注于固体口服制剂的开发,分析及临床供应。主导、参与或支持超过30个新药研发项目,其中5个实现商业化。
学历背景:清华大学高分子材料与化学工程学士 ,美国塔夫茨大学化学工程硕士,美国里海大学化学工程博士。
海外活动
02
Formulation Forum Webinar
Applying PBPK and PBBM Across R&D: From Early Insights to Formulation
9/24 ET | Online
Date/Time: September 24, Wednesday, 2:00-3:00 PM
Presenter:
Deanna Mudie, Ph.D.
Senior Principal Scientist of PBPK R&D
Simulations Plus
Abstract:
Scroll to read the full abstract
Register Link:
https://go.crystalpharmatech.com/crystal-pharmatech-formulation-forum/2025-ep11-registration
03
Simulation plus Webinar
Can you spare 25mg? Optimizing Benchwork and Computational Approaches to Assess CMC Success
9/11 ET | Online
Date/Time: September 11, 8:00 AM Pacific / 11:00 AM Eastern
Presenter:
Robert Wenslow, Ph.D.
Co-Founder and CBO
Crystal Pharmatech
Moderator:
Andrew Mueller
Director, Business Development
Simulations Plus
Summary
Drug discovery scientists often believe gram scale quantities of active pharmaceutical ingredient (API) are required to determine chemistry, manufacturing, and controls (CMC) properties like solubility, oral absorption, salt selection, and formulation strategy. But in this webinar, Crystal Pharmatech Co-Founder and Chief Business Officer, Robert Wenslow will present advanced computational approaches and a focused lab component to provide you with development risks with only 25mg of material. This allows developability to truly mainstream into late lead optimization and significantly enhance the probability of success (POS) for Phase I candidates.
Scroll to read the full summary
Attendees will have the chance to get their questions answered during the live Q&A moderated by Simulations Plus Director, Business Development, Andrew Mueller. Don’t miss out on this opportunity to learn from an industry expert—register now.
Register Link:
https://www.simulations-plus.com/events/can-you-spare-25mg-optimizing-benchwork-and-computational-approaches-to-assess-cmc-success/
04
American Drug Delivery & Formulation Summit
9/15-16 ET | Boston, MA
Date: September 15-16
Location: The Westin Copley Place, Boston
Attendee:
Ye Gu, Ph.D.
Co-founder, CTO and Head of Business Development
Crystal Bio Solutions (a member of Crystal Pharmatech)
Yongqiang Li, Ph.D.
CEO of Canada CDMO BU
Candoo Pharmatech
Dragutin Knezic, Ph.D.
Business Development Director
Crystal Pharmatech
Booth: 30
05
BioTechX USA
9/16-17 ET | Philadelphia, PA
Date: September 16-17, 2025
Location: Pennsylvania Convention Center, Philadelphia
Attendee:
Robert Wenslow, Ph.D.
Co-founder and CBO
Crystal Pharmatech
Derik McCarthy
Subject Matter Expert
Crystal Pharmatech
Booth: S5
Link:
https://www.terrapinn.com/conference/biotechxusa/index.stm







